We cover any subject you have. Which of the following elements would form ionic compounds.

The Ionic Bond Boundless Chemistry
Lone pair versus lone pair repulsion lone pair versus bonding pair repulsion bonding pair versus bonding pair repulsion.
. Thats our Place of Truth. There are two electron pairs around the central. The repeating units of proteins are a glucose units b amino acids c fatty acids d peptides 2.
In nature the organism with desirable characteristics. Essay Help for Your Convenience. Look over the writers ratings success rating.
Digitalis glycoside shown below is an organic. Get all these features for 6577 FREE. Amino acids are joined by a peptide bond b hydrogen bond c ionic bond d glycosidic bond 3.
Receive your papers on time. Intramolecular force and potential energy. This review paper describes carbon capture process using ILs mechanisms and challenges.
That is the two reactants each lose their. Which of the following statements best explains the Theory of Natural Selection. 2 pts BONUS Questions 1 pt.
Each 33 Given the reaction. 1091 The best writer. Valence electrons and ionic compounds.
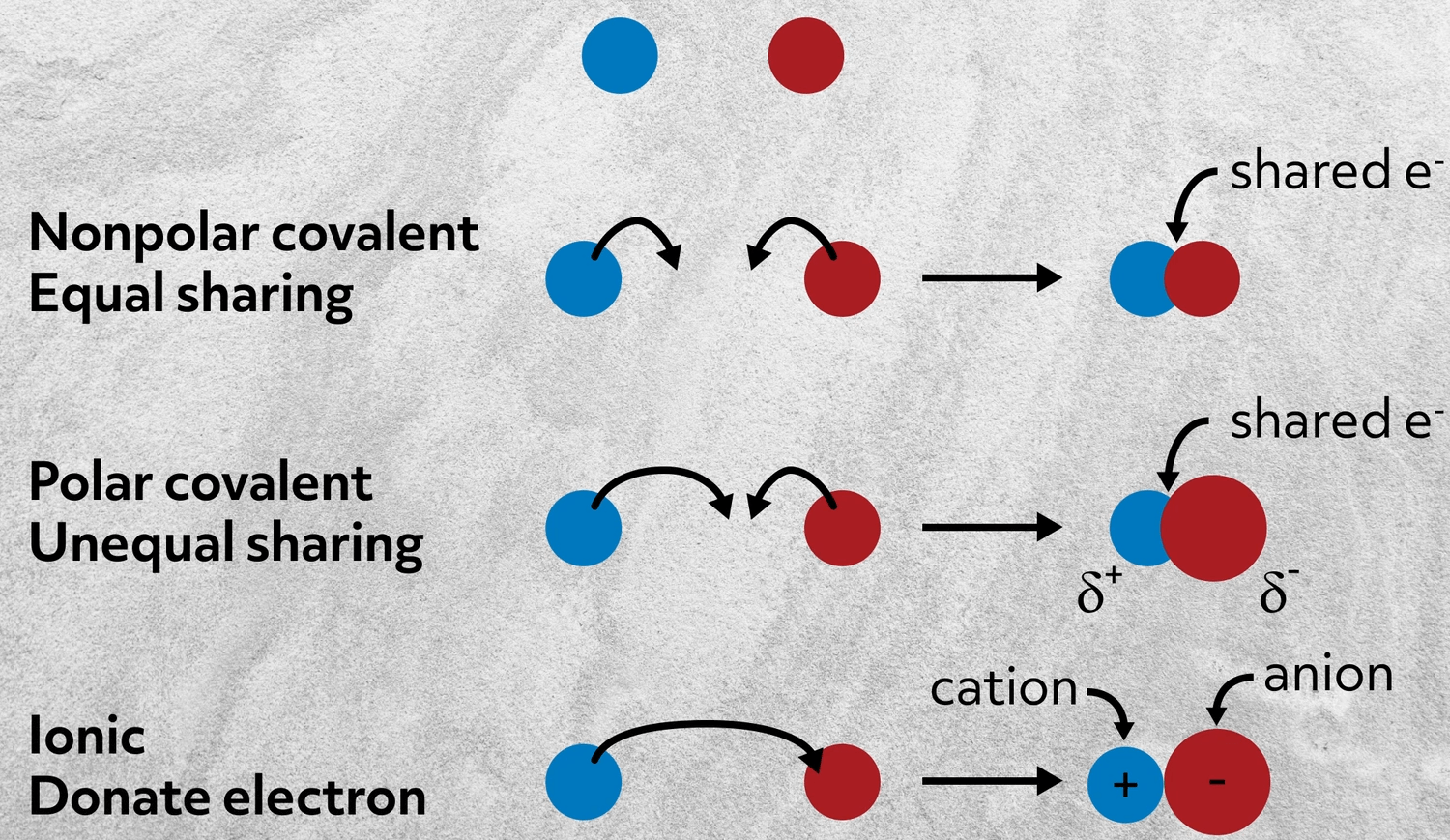
Any Deadline - Any Subject. The best guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in the periodic table. 79 of exam score.
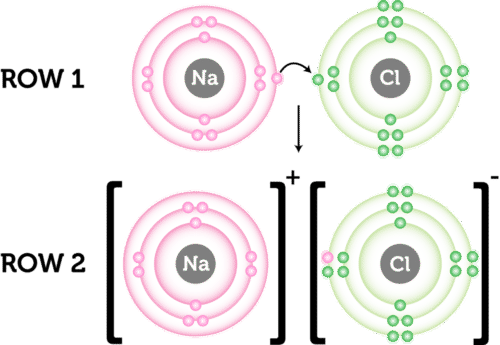
Sodium and magnesium. With such a theory we can say that ionic bonds are harder to break than covalent bonds. That quantity is known as bond dissociation energy.
CIt alters the reading frame of the mRNA. Types of chemical bonds. To elaborate if greater energy is required to break the bond the stronger that bond will be.
A It introduces a premature stop codon into the. CO 2 capture efficiency is studied at various conditions through parametric sensitivity analysis. Which of the following is a characteristic of alcohols.
The antioxidant-rich formula brings nourishment and shields against damage - both thermal and pollution. To use this key. 316 - 22 096.
B It changes an amino acid in the encoded protein. It can be used on all hair types but will work best for dry and damaged locks in order to restore shine and add moisture. A It introduces a premature stop codon into the mRNA.
Organs that are not used may disappear while organs that are constantly used may develop. This heat protection serum is designed to shield your tresses up to temperatures of 232C. H2 Cl 2 2HCl Which statement best describes the energy change as bonds are formed and broken in this reaction.
Bond angles are predicted in the following order. When the electronegativity difference is above the 04 polar covalent bond is formed. Which of the following alcohols may be safely consumed.
Here is a chart that describes the usual geometry for molecules based on their bonding behavior. The combining of elements to form different substances is called chemical bonding. Set the deadline and keep calm.
Which of the following statements correctly describes the effect a nonsense mutation would have on a gene. This can be thought of as a double displacement reaction where the partners switching. Which of the following involves the weak attraction of hydrogen from one molecule of water to oxygen from another molecule of water.
This paper discusses about different characterization tools for ILsCO 2 systems. D It has no effect on the amino acid sequence of the encoded protein. They are found in wine beer and distilled drinks.
Well the simple method that we can follow to determine this is by measure the energy required to break the bond. The primary structure of protein represents a Linear sequence of amino acids joined by peptide bond b 3-dimensional structure of protein c helical structure of protein. Carbon and hydrogen.
Likewise the Na and Cl atoms in NaCl have an electronegativity difference of 21 and the Mn and I atoms in MnI 2 have a difference of 10 yet both of these substances form ionic compounds. Thats why electron pair attracted more towards oxygen thus oxygen becomes partial negative and hydrogen becomes partial negative and polar covalent bond is formed. A The forming of the H-Cl bond releases energy.
Molecular and Ionic Compound Structure and Properties Youll discover the range of chemical bonds and how their structure can affect the properties of the molecules created. The ions replace each other based on their charges as either a cation or an anion. While if it is 04 or less than 04 non polar covalent bond is formed.
A common alcohol seen in the home is rubbing alcohol which is typically labeled with the common name_____ on the bottle. 32 Explain in terms of electronegativity why an H-F bond is expected to be more polar than an H-I bond. Take A Real Quiz.
CO 2 capture with ionic liquids ILs appears to be a promising strategy in the future. A double replacement reaction occurs when two ionic reactants dissociate and bond with the respective anion or cation from the other reactant. 50 points- Youd better try hard.
The lightweight product.

Ionic Bond Definition Properties Examples Facts Britannica
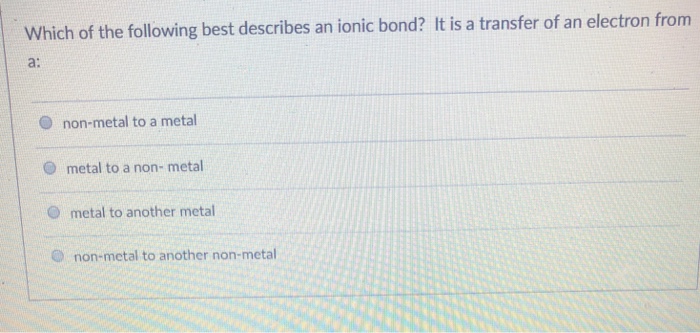
Solved Which Of The Following Best Describes An Ionic Bond Chegg Com
0 Comments